Quality
Quality, best practise and customer service - there's nothing more important to us. That's why we keep investing in our infrastructure, technology and people
Everything we do is focused on quality, best practise and customer service and we strive to lead the industry in all three. Our quality department is fully-equipped with quality control procedures throughout the production process so we can aim for complete customer satisfaction every time.
Our employees play a vital role in ensuring quality at every stage. We're committed to developing a culture of total quality management (TQM) inside our business. And for almost a decade we've been certified to hold ISO 9001 certification for quality. We use a range of procedures and equipment to ensure quality control and continuous improvement at every stage of our work.
We're proud to be an ISO 13485:2016 certified company. This is the industry standard for quality management systems for the medical devices industry and means we meet all appropriate ISO 9001 and FDA regulatory requirements that our customers and the regulators rightly demand.
Not every company can become certified for ISO 13485. To achieve it, we have demonstrated that we comply with a whole range of special requirements and systems:
Read more about our technical expertise or to discuss your next project with a member of our team, get in touch.
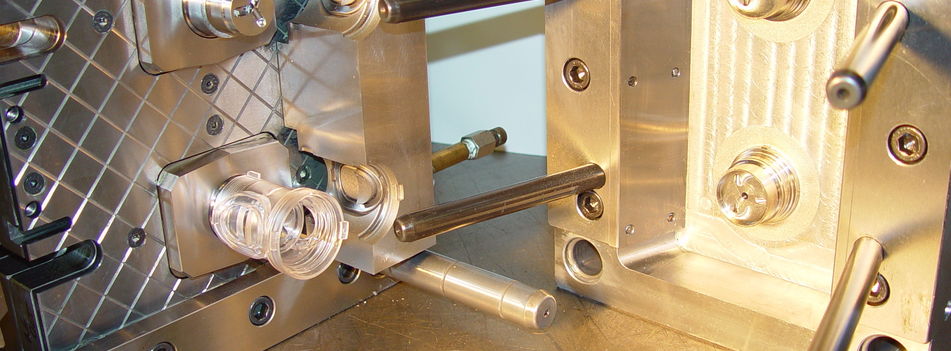
Many of the mould tools we run in our moulding machines have complex cores which operate in a plane 90 degrees to the tool opening plane.